Abstract
Introduction The SARS-CoV-2 pandemic risked significant disruption to haematopoietic cell transplantation (HCT). This led to changes in registry operations including widespread use of cryopreservation, Covid-19 screening strategies and deferral periods for positive donors or close contacts.
Anthony Nolan (AN), along with UK Aligned Registry partners were some of the few organisations that instigated donor SARS-CoV-2 testing across the donor pathway from April 2020 and are in a unique position to comment on UK stem cell donor prevalence. SARS-COV-2 PCR testing of donor was performed at medical, ahead of G-CSF or BM for cryopreserved collections, before conditioning for fresh collections, and day of donation, in keeping with NICE guidance. Early guidance from JPAC recommended postponing donation three months from resolution of symptoms in donors with coronavirus, with discretionary earlier clearance depending on individual risk assessment and urgency of transplant. Deferral periods were subsequently reduced to 14 days. AN followed public health advice from UK government which included mandatory quarantine for contacts (lifted for vaccinated individuals from 16/8/21) and confirmed cases (legal requirement lifted 24/2/22).
Methods Here we report for the first time our 2-year experience of the impact of Covid-19 on our large UK stem cell registry including the SARS-COV-2 prevalence in the UK donors, and impact on collections and cell infusion. This retrospective study included all UK donors (AN and BBMR) selected by UK and International Transplant centres (TCs) to donate PBSC, BM or DLI for paediatric and adult patients between 1st July 2020 and 1st July 2022. This does not include positive donors at verification typing stage or ahead of being selected as preferred donor.
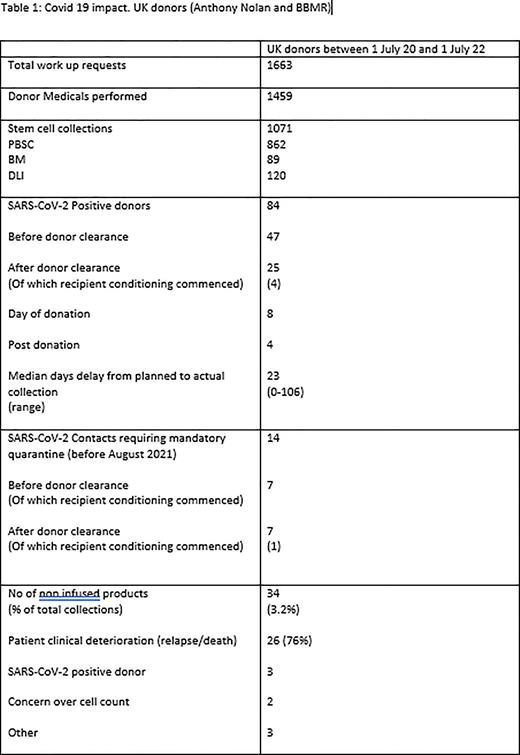
Results During study period AN received 1663 work up request for UK donors with 1459 of these donors attending a planned medical. There were 1071 collections (862 PBSC, 89 BM, 120 DLI). 709 (66.2%) of these were cryopreserved.
84 donors tested SARS-CoV-2 positive after being selected and scheduled as a donor giving a prevalence in this two-year period of 5.8%. This occurred before donor clearance was sent to TC in the majority of cases, 47 (56%). 25 (29.8%) occurred after clearance, of these 4 were due to be fresh collections with the intended recipients having commenced transplant conditioning (2 held conditioning and used same donor, 2 used back up donor). There were 8 donors positive on day of donation (7 PBSC, 1 DLI) amounting to 0.75% of all collections. Products were infused in 5 cases (3 fresh, 2 cryo) and discarded in three cases (all cryopreserved). 4 donors were positive within 2 days post donation. We are not aware of any complications or transmitted SARS-CoV-2 from the infused cells. 14 additional donors required mandatory isolation for Covid contact: one of which at a late stage after recipient had commenced conditioning, making a total of 5 post-conditioning cancellations/postponements.
For the 84 confirmed SARS-CoV-2 donors, 49 (58.3%) went on to donate with a median deferral from time of original planned collection of 23 days (range 0-106 days). 11 were cancelled for alternative reasons and 24 (28.6%) opted for an alternative donor.
During the study period there were a total 34 cryopreserved collections from UK donors not infused (3.2% of UK collections) most commonly due to change in patient circumstances (deterioration or death, 76%).
Conclusion AN significantly adapted working during the Covid-19 pandemic. Both cryopreservation and the testing strategy were integral to ensuring the safety of our donors, recipients and collection centre staff and procedures. Covid-19 infection in selected donors (5.6%) led to collection delays (average 23 days) but, whilst each individual case was unfortunate and regrettable, the fact that this transpired to only 5 late-stage (post-conditioning) cancellations/postponements during the study period, does provide reassurance. This highlights during times of high community prevalence having a back-up donor option for planned fresh infusions remains valid advice, and if this cannot be assured then cryopreservation is likely to remain a valuable option. The high number of non-infused products is an area of concern and highlights importance of effective communication between TC and registry, and the importance of completing pre-transplant work up assessment ahead of donor mobilisation.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal